Technical Guidelines
45 technical guidelines
 Pandemic Preparedness Plan (PPP)
Pandemic Preparedness Plan (PPP) Report of the Regional Workshop on Lessons Learned During the COVID-19 Pandemic in the Region of the Americas: Preparedness and Response. Buenos Aires, 16–19 August 2022
 Laboratory
Laboratory Samples from patients suspected of Influenza A/H5 Laboratory Testing Algorithm
 Laboratory
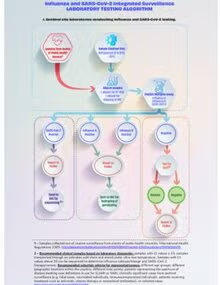
Laboratory Influenza and SARS-CoV-2 integrated surveillance laboratory testing algorithm
 Surveillance
Surveillance Influenza and Other Respiratory Viruses: Surveillance in the Americas 2021
 Surveillance
Surveillance Final report Ad hoc expert consultation in the Region of the Americas: Challenges, gaps and next steps in COVID 19 surveillance and its integration into influenza and other respiratory viruses surveillance
 Pandemic Preparedness Plan (PPP)
Pandemic Preparedness Plan (PPP) Strengthening Pandemic Preparedness Planning for Respiratory Pathogens Policy Brief (New)
 Surveillance
Surveillance End-to-end integration of SARS-CoV-2 and influenza sentinel surveillance: revised interim guidance
 Clinical management
Clinical management Guidelines for the clinical management of severe illness from influenza virus infections
 Prevention and control
Prevention and control Highlights from the Meeting of the Strategic Advisory Group of Experts (SAGE) on Immunization, 2021
 Pandemic Preparedness Plan (PPP)
Pandemic Preparedness Plan (PPP) PIP PC Preparedness High-Level Implementation Plan II 2018-2023
 Animal-human interface
Animal-human interface Joint Risk Assessment Operational Tool (2021)
 Prevention and control
Prevention and control Readiness for influenza during the COVID-19 pandemic: policy brief, 6 November 2020
- ‹ Previous
- 1
- 2
- 3
- 4
- Next ›